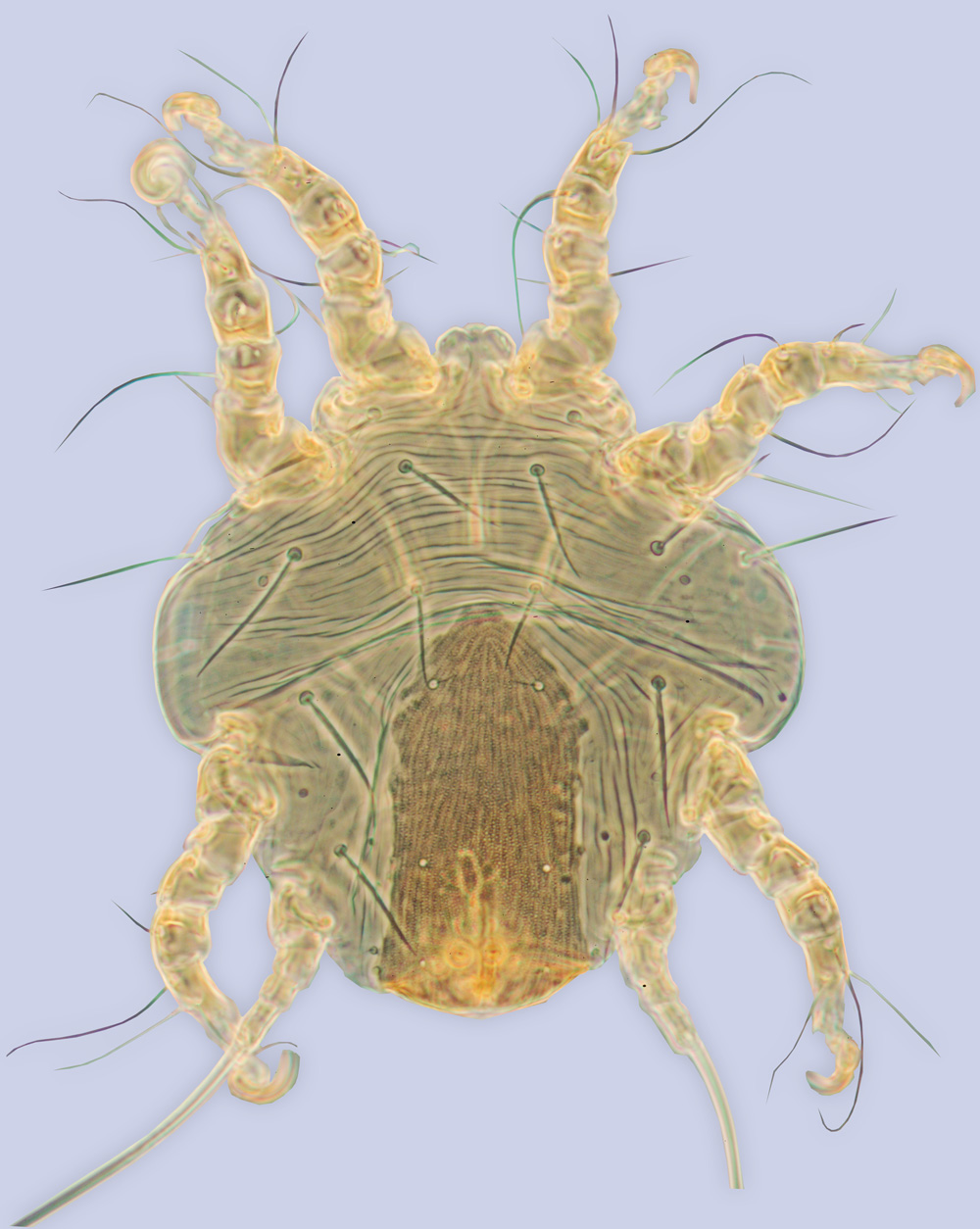
Fig. 1. Phoretic deutonymph of Sennertia americana, ex Xylocopa virginica, Virginia (BMOC 90-1212-025).
Click here to enlarge
|

Fig. 2. Phoretic deutonymphs of the mite Sennertia americana dispersing on the carpenter bee, Xylocopa virginica virginica, Illinois (BMOC BMOC 04-1222-159).
Click here to enlarge
|
Bee Mites : Acari : Acariformes : Sarcoptiformes : Chaetodactylidae : Sennertia : Sennertia species
Sennertia americana Delfinado and Baker, 1976
Sennertia americana Delfinado & Baker, 1976: 84, Figs 31-32 (Holotype HDN in and 59 paratype HDNs in USNM; original type repository NYSM (holotype), NYSM and USNM (paratypes)); OConnor, 1988: 341; OConnor, 1993b: 164; Ramaraju & Mohanasundaram, 2001: 107; Klimov & OConnor, 2008: 173, Figs 88-89; Okabe et al., 2008: 1378
Sennertia (Amsennertia) americana: Fain, 1981a: 147; Alzuet & Abrahamovich, 1987: 346; Lombert et al., 1987: 113, Figs 1-30 (description of ontogeny); OConnor, 1993a: 362 (acquisition of genus-level characters)
Material (show database records) (all from Xylocopa virginica). 26 HDNs - USA: Florida, Alachua Co., Gainesville, (propodeum), 10 May 1924, L. E. Jeffries, UMMZ BMOC 04-0917-001; 13 HDNs - Chipola Lake, male (propodeum), 9 Apr 1927, no collector, CUIC HK 85-0108-003; 11 HDNs (paratypes) - Florida, Lee Co., female, 28 Feb. 28, 1937, KVK, USNM; 16HDNs - Illinois, Macoupin Co., Carlinville, Crataegus mollis (T. & G.) Scheele (Rosaceae), on propodeum & 1st metasomal tergite, 26 Apr 1971, J. C. Marlin, INHS Insect Collection 62498, BMOC 04-1222-158; 10HDNs – same locality, Prunus serotinus Erhart (Rosaceae), 1st metasomal tergite, 9 May 1971, J. C. Marlin, INHS Insect Collection 62499, BMOC 04-1222-159; 11 HDNs - Maryland, Baltimore Co., Baltimore, (propodeum), 28 Apr 1957, R.G. Beard, CUIC BMOC 79-1205-002; 5 HDNs - Michigan, Washtenaw Co., Ann Arbor, Green Brier Apts, 24 May 2000, A. Dowling, UMMZ BMOC 04-1008-001; New York, 6 HDNs (1 holotype and 10 paratypes) - Albany Co., Albany, 6 Jun 1901, no collector, USNM; 15 HDNs Dutchess Co., Amenia (propodeum), 9 May 1978, M. O'Brien, UMMZ BMOC 04-0917-002; 28 HDNs (paratypes) - Dutchess Co., Poughkeepsie, Apr 1901, no collector, USNM; 4 HDNs - Tompkins Co., Ithaca, Cornell Campus, (near wing base), 4 Sep 1975, R. J. Pollack, UMMZ BMOC 76-1017-002; 10 HDNs - North Carolina, Craven Co., Fairfield Harbour, 15 ft., marsh/woods (dorsolateral propodeum), 9-11 May1994, D.C. Marshall, UMMZ BMOC 94-1104-001; 1 female, 2 males, 1 TN - Pennsylvania, Huntingdon Co., Marklesburg, nest, 1 Aug 1981, R. Fisher, UMMZ BMOC 82-0521-019; 1 L, 2 PNs, 1 HDN, 1 TN, 3 females, 3 males - same data, USNM; 1 pharate HDN - Texas, Dallas Co., ex X. v. texana (mesosoma), 1 Jul 1931, J.K.G. Silvey, UMMZ BMOC 90-1212-020; 17 HDNs - Virginia, Amherst Co., Sweet Briar Station, (propodeum ), 27 Apr 1938, E. Herbold, UMMZ BMOC 90-1212-025; 4 HDNs - North America, male (propodeum), no date, R. Latham, CUIC HK 85-0108-004. Voucher specimens in CNC, CUIC, INHS, OSAL, UMMZ, UNAM, USNM.
Description. Phoretic deutonymph. Gnathosomal solenidia shorter than 1/3 of femur I width. Supracoxal setae scx situated on separate small sclerite. Hysterosomal shield lateral gland openings and bases of f2 nearly on edge of hysterosomal shield, or the former outside the shield. Lateral edges of hysterosomal shield in anterior part not narrowing. Dorsal hysterosomal pouch absent. Distance between anterior margin of hysterosomal shield and setae si exceeds diameter of si bases. Striate pattern of idiosomal cuticle outside hysterosomal shield without sclerotization, formed by long striae. Distinct rudiments of vi present. Setae si distinctly posterior se, exceed 1/2 of se, almost as thick as se. Diameter of si exceeds 1/2 of diameter of se. Setae c1 distinctly longer than d1-h1; long, nearly as long as se; situated anterior to hysterosomal shield. Setae d1 and e1 nearly uniform in length with h1. Setae d1 shorter than 1/4 of distance between them; situated on hysterosomal shield. Sclerite between ia and d2 absent. Setae e2 subequal with d2; not touching hysterosomal shield. Lateral gland openings situated outside hysterosomal shield. Setae 4b, g, and 4a without distinct rhomb-like widening, filiform. Setae 4b, pR I-II, sR III, wF IV, gT I-II, hT I-II, kT III, ra I-II, and wa I-II filiform. Posterior apodemes II and anterior apodemes III free. Posterior processes of coxal apodemes IV non-applicable. Anterior apodemes IV not interrupted, almost straight. Posterior apodeme IV absent. Conoids ps2 posterior to anterior transverse level of central suckers (ad1+2); anterior to ps1, situated outside outer level of ad1+2. Transparent margin of anterior suckers (ad3) without rough sclerotization. Suckers ad3 not enlarged, smaller than central suckers. Posterior and lateral borders of attachment organ not forming distinct frame. Sclerotized rudiment of anterior cuticular suckers present. Longitudinal hysterosomal sclerite present, long. Ventral hysterosoma smooth. Genual setae mG I-II simple, mG II almost as long as leg II or longer. Tarsal setae la I-II longer than famulus ε. Tarsal setae ra I-II not bifid, filiform. Tarsal setae wa I-II and s III filiform, needle-like, or widened basally but with attenuated end. Tarsal setae d I-II slightly widened. Tarsal setae d and f I-II almost symmetrical, not touching. Solenidion ω3 closer to f I than to ω1. Posterior condylophore present. Anterior condylophore I-II with distal bending. Seta d III situated close to tarsal base, distance usually subequal or shorter than diameter of d III alveolus. Leg IV protruding posterior edge of hysterosoma. Tarsus IV not enlarged, shorter or less than 2 times longer than width of trochanter IV. Setae w IV thinner than d IV and distinctly shorter than leg IV, situated on middle of tarsus IV. Setae s IV present. Setae wF IV distinctly protruding apex of tarsus IV.
Adults. Supracoxal seta scx situated on supracoxal sclerite, anterior to outer ridge of supracoxal sclerite. Alveoli of setae ve absent. Dorsal idiosomal cuticle tuberculate or mammillate (except for posterio-medial opisthosomal region in female). Dorsal cuticular pattern more or less uniform. Dorsal idiosomal setae c1-h1 spiniform, short (not reaching half of distance to next posterior pair of setae). Dorsal idiosomal setae cp, c3, h3 widened distally, compressed dorso-ventrally at apex, barbs more numerous at apex. Dorsal setae e2 and f2 spiniform, distinctly shorter than h3. Prodorsal shield distinctly elongated, length/width 1.7-1.8, without falsifoveate pattern. Coxal fields III opened. Proximal acetabular extensions of ap' I partially border antiaxial margins of coxal fields I. Proximal acetabular extensions of ap' II partially border antiaxial margins of coxal fields II. Distal acetabular extensions of ap' II and ap'' II separate. Proximal acetabular extensions of ap' III completely border antiaxial margins of coxal fields III. Distal acetabular extensions of ap' III and ap'' III fused. Proximal acetabular extensions of ap' IV completely border antiaxial margins of coxal fields IV. Distal acetabular extensions of ap' IV and ap'' IV separate or not developed. Opisthosomal gland openings approximately at level of e2. Tarsal setae ra and la II present. Solenidion ω2 I subapical. Famulus ε spiniform. Setae ba I longer than famulus ε. Setae ba II absent.
Female. Setae ad1 and ad2 absent. Setae ps3 short, distinctly shorter than ps2; anterior to 4a level. External copulatory tube absent. Setae h3 nearly at level of h2. Posterio-medial part of dorsal opisthosoma with distinct longitudinal linear pattern.
Male. Setae ad1 absent. Genital setae short, transparent mammillae; situated on progenital folds. Pseudanal setae ps3 outside progenital sclerites, spiniform. Dorsal supporting sclerites short, as long as 2 diameters of aedeagus at base or shorter. Setae q I present, p II absent. Pretarsal suckers IV same as pretarsal suckers I-III.
Protonymph. Tarsal setae e IV absent, f IV absent.
Larva. Dorsal idiosomal setae relatively longer than in other instars, protruding bases of subsequent setae.
Hosts. Xylocopa (Xylocopoides) virginica (type host), Xylocopa (Xylocopoides) virginica texana.
Distribution (Show map). USA: Florida, Illinois, Maryland, Michigan, New York (Albany Co., Albany - type locality), North Carolina, Pennsylvania, Texas, Virginia.
Biology. Feeding instars of this species were found only once (Lombert et al. 1987). At the time these collections were made in early August, adult Xylocopa virginica were emerging from the nests, and most cells were already empty. A few cells contained pharate or teneral adult bees. Some cells contained large provision masses consisting of nectar and pollen, but no developing bee larvae. All instars of Sennertia americana were located on the walls of these cells. The cells were also inhabited by Horstia virginica Baker, 1962 (Acaridae) and Tortonia quadridens Baker, 1962 (Suidasiidae). Since the two latter mite species are cleptoparasitic the ultimate cause of cell failure is uncertain.
Note. The slide marked "holotype" contains six specimens none of which is identified as the holotype.
References
Alzuet, A. B. d. & A. H. Abrahamovich. 1987 [1985]. Deutoninfas (hypopi) de los géneros Sennertia Oudemans, 1905 y Horstia Oudemans, 1905 (Acari: Astigmata) sobre Xylocopa (S.) splendidula splendidula Lepeletier, 1841 (Hymenoptera: Apoidea). Revista de la Sociedad Entomologica Argentina.44: 345-351.
Delfinado, M. D. & E. W. Baker. 1976. Notes on hypopi (Acarina) associated with bees and wasps (Hymenoptera). Journal of the New York Entomological Society.84: 76-90.
Fain, A. 1981. A revision of the phoretic deutonymphs (hypopi) of the genus Sennertia Oudemans, 1905 (Acari, Astigmata, Chaetodactylidae). Systematic Parasitology.3: 145-183.
Klimov, P. B. & B. M. OConnor. 2008. Morphology, evolution, and host associations of bee-associated mites of the family Chaetodactylidae (Acari: Astigmata), with a monographic revision of North American taxa. Miscellaneous Publications Museum of Zoology University of Michigan.199: 1-243.
Lombert, H. A. P. M., B. M. OConnor, F. S. Lukoschus & J. O. Whitaker, Jr. . 1987. Ontogeny, systematics and ecology of Sennertia (Amsennertia) americana Delfinado & Baker, 1976 (Acari: Chaetodactylidae) from the nest of the carpenter bee, Xylocopa virginica (Hymenoptera: Anthophoridae). International Journal of Acarology.13: 113-129.
OConnor, B. M. 1988. Coevolution in astigmatid mite-bee associations. In African honey bees and bee mites, eds. G. R. Needham, R. E. Page, Jr., M. Delfinado-Baker & C. E. Bowman, 339-346. Chichester, New York, Brisbane etc.: Ellis Horwood Ltd & John Wiley & Son.
OConnor, B. M. 1993a. Generic relationships in the Chaetodactylidae (Acari: Astigmata) with description of a new genus. Acarologia.34: 345-362.
OConnor, B. M. 1993b. The mite community associated with Xylocopa latipes (Hymenoptera: Anthophoridae: Xylocopinae) with description of a new type of acarinarium. International Journal of Acarology.19: 159-166.
Okabe, K., S. I. Makino & T. Endo. 2008. Polymorphism in the deutonymph and adult of Sennertia alfkeni (Acari: Chaetodactylidae) associated with the large carpenter bee, Xylocopa appendiculata circumvolans (Hymenoptera: Apidae). Journal of Natural History.42: 1361-1384.
Ramaraju, K. & M. Mohanasundaram. 2001. New phoretic mites (Acari: Chaetodactylidae) on carpenter bees from Tamil Nadu, India. International Journal of Acarology.27: 107-112.
B. OConnor and P. Klimov ©
Created: May 26, 2011
Last modified: 
